
We’ve been waiting for this.
THIS IS BIG NEWS IN THE WORLD OF COSMETIC SURGERY AND AESTHETICS!!!
The first injectable cellulite treatment approved in USA and news that has made 2020 worthwhile!!!! #gamechanger

There’s finally another in-office option for the stubborn cellulite that gives many of our bodies that orange-peel look, making us crazy during bathing suit season (right now!). Topical products can only do so much—they can’t penetrate deep enough and also don’t have the power to break up the cellulite-causing bands—and the in-office treatments that are currently available utilize more invasive methods to reach and cut the bands for smoother skin.
But, Endo Aesthetics just announced that it has received FDA approval for Qwo (collagenase clostridium histolyticum-aaes), making it the first and only injectable treatment indicated to treat moderate-to-severe cellulite on the buttocks in adult women.
According to the brand, when injected into the treatment area, Qwo is thought to release the fibrous septae enzymatically by specifically targeting Types 1 and 3 collagen, which may result in smoothing of the skin and an improved appearance of cellulite. However, it’s important to note that no cellulite treatment is a magic bullet, and like with any injectable, there may be some bruising and pain at the injection site.
“Endo recognized a significant unmet need for an effective injectable treatment for cellulite, which led us to conduct the largest clinical trials in the history of cellulite investigation in the United States,” says Dr. Matthew Davis, senior vice president and chief medical officer for Endo. “Supported by rigorous research, testing and development processes, we are proud to have received FDA approval of the first injectable treatment for cellulite in the buttocks, and we look forward to delivering Qwo to the aesthetics community and their adult female patients.”

New York dermatologist Marnie Nussbaum, MD says cellulite has long been a hot button topic, and there are major misconceptions about how we get it.
“More than 90 percent of women will experience cellulite in their lifetimes: 36 percent is hereditary and the rest is related to hormones. And as we age and our skin laxity increases on areas like the thighs and butt, it makes cellulite dimples even more visible. When we eat unhealthy and you gain weight, it makes the cellulite look worse, but the cellulite itself doesn’t get worse—the fat pushes the cellulite out. If you think about it, losing weight doesn’t take it away.
Men, on the other hand, have a completely different orientation of their muscle-to-fat ratio and skin cells, and they don’t have estrogen, so it doesn’t affect them the same way.”
The primary end point of the study was the proportion of 2-level multicomponent responders at Day 71 post randomization defined as at least 2 levels of improvement in cellulite severity from baseline on both the Clinician Reported Photonumeric Cellulite Severity Scale (CR-PCSS) and Patient Reported Photonumeric Cellulite Severity Scale (PR-PCSS).
Results from both studies showed treatment with Qwo was associated with greater reductions in cellulite severity compared with placebo as measured by CR-PCSS and PR-PCSS scales at day 71. Additionally, both studies showed that treatment with Qwo led to greater improvement in the measure of patient-reported satisfaction compared with placebo.
“[The] FDA approval of QWO is a key achievement in the continued execution of Endo’s long-term strategy, especially as it relates to building our portfolio and capabilities for the future,” said Blaise Coleman, President and Chief Executive Officer of Endo. “As Endo embarks on an exciting new journey into medical aesthetics, we look forward to bringing this innovative treatment to market through our Endo Aesthetics organization.”
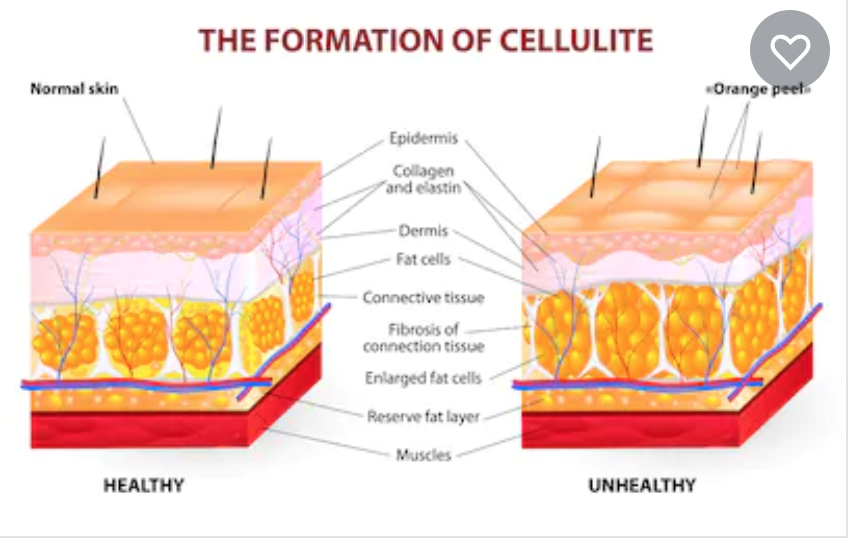
While cellulite is known to be a multifactorial condition, a primary contributing factor is the fibrous connective tissue, called the “fibrous septae,” which connect the skin perpendicularly to the fascia below.2,3 These fibrous septae tether the skin, drawing it downward and leading to a mattress-like appearance, commonly referred to as “dimpling.”4,5 When injected into the treatment area, QWO is thought to release the fibrous septae enzymatically by specifically targeting Types 1 and 3 collagen, which may result in smoothing of the skin and an improved appearance of cellulite.

“Endo recognized a significant unmet need for an effective and non-invasive injectable treatment for cellulite, which led us to conduct the largest clinical trials in the history of cellulite investigation in the United States,” said Matthew Davis, M.D., R.Ph., Senior Vice President and Chief Medical Officer of Endo. “Supported by rigorous research, testing and development processes, we are proud to have received FDA approval of the first injectable treatment for cellulite in the buttocks and we look forward to delivering QWO to the aesthetics community and their adult female patients.”
Side effects of QWO included injection site bruising, pain, areas of hardness and itching in the treatment area.
“QWO could be a game-changer for many women with cellulite,” said Anne Chapas, M.D., a board-certified dermatologist at Union Square Laser Dermatology in New York City. “I am thrilled there will now be an FDA-approved injectable treatment option proven to address a root cause of cellulite. What is exciting about QWO is that it is a cutting-edge cellulite treatment, without the cutting.”
Credit to Aesthetic Medical Practitioner News for the original story.





















